APEX C: Carbon rigid wheelchair
The APEX C Carbon Rigid Wheelchair is the ultimate performance chair by Motion Composites, utilizing advanced carbon fiber technology for maximum strength and agility. LIVE BOLDLY WITHOUT LIMITS.

The APEX Carbon wheelchair by Motion Composites belongs to a new era of rigid wheelchairs. A balanced blend of technology and design, it is the ultimate in lightness, durability, flexibility and style. It combines high-end technology and all the advantages of carbon fiber in a modern-looking, fully adjustable chair. This model is a Red Dot Award winner for its outstanding design.
APEX Carbon Wheelchair Key Benefits

Unparalleled Lightness
Made of the industry’s most advanced materials, the APEX Carbon is state-of-the-art. A transport weight of just 9.8 lbs ensures agile movement and easy transportation.

Optimized Handling
The unique rigidizing bar maximizes stability while preserving reactivity. This sturdiness reduces lateral movement and offers optimal propulsion for greater mobility.

Perfected Materials
Carbon fiber ensures exceptional durability. It has superior fatigue resistance and can withstand extreme temperatures for a chair that will last for years.
APEX Carbon Wheelchair Features
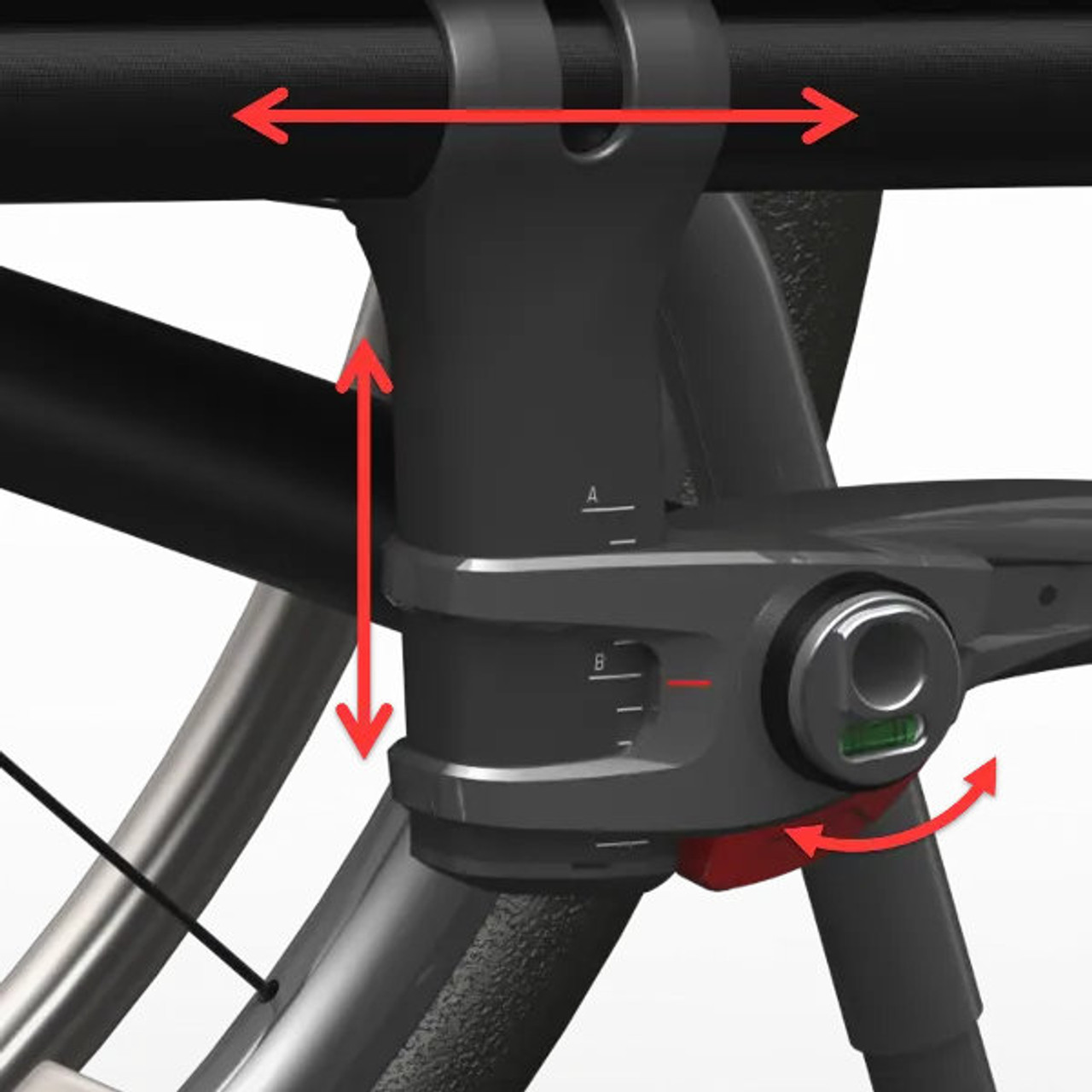
- Back Rest Adjustment: Offers a wide range of adjustments to fit any position.
- Carbon Rigidizer Bar: Maintains strength and durability through the life of the chair.
- Integrated Impact Guard: Prevents daily use scratches and adds a high-friction grip for transfers.
- Standard Carbon Fiber Camber Tube: Adds strength and reduces overall weight.
- Rear Axle Plate: Provides a multitude of adjustments while maximizing stability and responsiveness.
- Bubble Levels: Built-in gauges make caster adjustments quick and precise.
APEX Carbon Wheelchair Specifications
APEX Carbon Measuring Guide
APEX Rigid Wheelchair Video
APEX C Carbon Rigid Wheelchair, by Motion Composites Buyer’s Guide
The APEX C Carbon Rigid Wheelchair, by Motion Composites is a high-performance mobility device constructed from premium carbon fiber for maximum strength, agility, and reduced weight. Engineered for responsive handling and customized fit, this rigid wheelchair is ideal for users who demand precision and efficiency in daily and athletic environments.
Lightweight Carbon Fiber Frame Design
The APEX C Carbon Rigid Wheelchair utilizes a carbon fiber frame that offers exceptional weight-to-strength ratio. This advanced material reduces the chair’s overall weight without compromising durability, resulting in a more agile and energy-efficient ride.
APEX C Carbon Rigid Wheelchair Maximum Responsiveness
Designed with a fully rigid frame, the APEX C provides optimal energy transfer with each push. This rigidity minimizes frame flex, allowing for improved propulsion and maneuverability whether navigating indoor spaces or traversing uneven outdoor terrain.
Motion Composites APEX C Adjustable Center of Gravity
The APEX C Carbon Rigid Wheelchair features a customizable center of gravity, allowing users to fine-tune the chair’s responsiveness and balance. This configuration supports a variety of seating preferences and activity levels, from casual everyday use to performance-driven mobility.
Integrated Monocoque Frame Construction
The carbon monocoque construction of the rigid wheelchair integrates multiple structural components into one seamless frame. This design increases strength, reduces weight, and improves overall structural integrity while maintaining a sleek and minimal profile.
APEX C Carbon Rigid Wheelchair Ergonomic Seating Options
The APEX C wheelchair offers several ergonomic seating systems, including tension-adjustable backrests and seat upholstery. These features help maintain proper posture and distribute weight evenly, reducing pressure points during extended use.
Compact Transport and Storage
Although built for performance, the APEX C is also designed for convenience. The quick-release rear wheels and folding backrest allow the chair to be easily disassembled for transport or storage in tight spaces such as car trunks or closets.
Performance Tires and Casters
High-quality tires and casters are included with the APEX C rigid wheelchair for maximum rolling efficiency and shock absorption. These components provide a smooth, stable ride and excellent traction on a variety of surfaces, enhancing user control and confidence.
APEX C Carbon Rigid Wheelchair, by Motion Composites Review
The APEX C Carbon Rigid Wheelchair, by Motion Composites is consistently rated for its advanced materials, responsive handling, and customizable features. Users appreciate its ultralight frame, rigid performance, and sleek design that meets both lifestyle and performance needs. This chair remains a leading choice for active wheelchair users seeking high-end mobility solutions.
No Buyers info available
PHONE 1-866-650-6555 FAX 1-888-966-6555 EMAIL info@motioncomposites.com WEBSITE motioncomposites.com
0070514E-00-V1-LIMITED WARRANTY USA - ENGLISH
REVISED ON 2025-06-04
1.1 MOTION COMPOSITES LIMITED WARRANTY – USA
A. CARBON PARTS – LIMITED LIFETIME
Motion Composites warrants the carbon fiber wheelchair frame, carbon fiber cross-brace, carbon fiber backcanes, carbon fiber sideguards, and carbon fiber footplates against defects in materials and workmanship for life. This warranty is limited to the carbon fiber frame, carbon fiber cross-brace, carbon fiber backcanes, carbon fiber sideguards, and carbon fiber footplates, and is limited to the first user and is not transferable.
B. ALUMINUM FRAME AND CROSS-BRACE – LIMITED LIFETIME
Motion Composites warrants the Aluminum wheelchair frame and Aluminum cross-brace against defects in materials and workmanship for life. This warranty is limited to the Aluminum frame and the Aluminum cross-brace and is limited to the first user and is not transferable. The expected life of the frame and cross-brace is five years.
1.2 COMPONENTS – 1 YEAR
- Motion Composites warrants all Motion Composites-made components, including the adjustable tension back upholstery, cushions and seat upholstery, against defects in materials and workmanship for limited one (1) year from the date of purchase, except for parts listed below.
- Motion Composites covers the following items for 30 days: tires and tubes for front or rear wheels, seat slings, standard back upholstery, armrest, push-handle grips;
- This warranty does not cover: damage arising from normal wear and tear or from other circumstances beyond Motion Composites’ control.
- The foregoing warranty shall not apply if:
- The original Motion Composites serial number tag has been removed, altered or defaced;
- Or the wheelchair has been subjected to negligence, accident, improper maintenance, storage or operation as required by your Motion Composites Owner’s Manual, commercial or institutional use, misuse or abuse, including, but not limited to, exceeding the maximum weight capacity of 265 pounds (120 kg), or 350 pounds (159 kg) if equipped with HD Kit;
- Or the wheelchair has been damaged by improper repairs or repairs made to any component without the express written consent of Motion Composites;
- Or the wheelchair has been modified without Motion Composites’ express written consent, including, but not limited to, modification using unauthorized parts or attachments;
- Or the wheelchair has been used as a weight training apparatus;
- Or the wheelchair’s Transit Tie-Down System (TTDS) has been misused; if TTDS is not attached to the four tarpaulin bows dentified and installed by Motion Composites.
- The shelf life or use life of the wheelchairs and their components could vary pending on the, practice, treatment, handling and frequency of use. Exposure to sun, heat, and water could affect the life of the wheelchair. Maintaining the wheelchair clean and storing it in a cool, dry area and away from the sunlight, will increase the duration of the wheelchair;
- This warranty is extended only to the original consumer purchasers of Motion Composites’ product.
- Devices and components subject to replacement or repair under one of these warranties remain subject to this warranty for its remaining term. However, components replaced or repaired under warranty within ninety (90) days of its expiration are guaranteed ninety (90) days, except those replaced or repaired under application of the thirty (30) days warranty within the thirty (30) days of its expiry, which shall then be guaranteed for thirty (30) days.
1.3 IMPORTANT NOTICE REGARDING CONSUMERS RIGHTS
The benefits we give in this manufacturer’s warranty are additional to, and do not detract from, any rights and remedies that you may have under local consumer protection laws.
This manufacturer’s warranty is governed by the laws of the country, province, state or territory in which you purchased your Motion Composites product.
In many countries, consumers have statutory rights under local consumer laws. Those consumer rights may differ between countries, territories, states or provinces, and often cannot be excluded.
This Manufacturer’s warranty is not intended to:
- change or exclude any statutory consumer rights that cannot be lawfully changed or excluded; or
- limit or exclude any right you have against the person who sold the Motion composites product to you if that person has breached their sales contract with you.
1.4 OUR RESPONSIBILITY
Motion Composites’ sole obligation and your exclusive remedy under this warranty shall be limited to such repair and/or replacement.
1.5 WARRANTY SERVICE
If your wheelchair requires warranty service, please contact an authorized Motion Composites Dealer in Canada or an authorized international distributor. In the event of a defect in material or workmanship, the Dealer or Distributor must obtain a return authorization (RA) number from Motion Composites. Motion Composites issues RA numbers only to authorized Motion Composites Dealers and Distributors. If you do not receive satisfactory warranty service, please write directly to Motion Composites Customer Service at
160 Armand Majeau Sud,
Saint-Roch-de-l’Achigan (Quebec) J0K 3H0 Canada
Phone: 1-866-650-6555
Fax: 1-888-966-6555
or send an email at: info@motioncomposites.com
Do not return products to our factory without our prior consent.
1.6 CONSUMER NOTICE
- The foregoing warranty is exclusive, and in lieu of all other express warranties, whether written or oral, express or implied. Motion Composites shall not be liable for any consequential or incidental damages whatsoever. By registering your Motion Composites wheelchair, you will be deemed to agree with all provisions of this warranty.
- It is forbidden to alter or extend the foregoing express warranty or to waive any of the limitations or exclusions.
LIMITED WARRANTY – USA
PHONE 1-866-650-6555 FAX 1-888-966-6555 EMAIL info@motioncomposites.com WEBSITE motioncomposites.com
0070514E-00-V1-LIMITED WARRANTY USA - ENGLISH
REVISED ON 2025-06-04
LIMITED WARRANTY – USA
1.7 MEDWATCH
MedWatch is the Food and Drug Administration’s (FDA) program for reporting serious reactions, product quality problems, therapeutic inequivalence/failure, and product use errors with human medical products, including drugs, biologic products, medical devices, dietary supplements, infant formula, and cosmetics.
If you think you or someone in your family has experienced a serious reaction to a medical product, you are encouraged to take the reporting form to your doctor. Your health care provider can provide clinical information based on your medical record that can help FDA evaluate your report.
However, we understand that for a variety of reasons, you may not wish to have the form filled out by your health care provider, or your health care provider may choose not to complete the form. Your health care provider is NOT required to report to the FDA. In these situations, you may complete the Online Reporting Form yourself.
You will receive an acknowledgement from FDA when your report is received. Reports are reviewed by FDA staff. You will be personally contacted only if we need additional information.
1.8 SUBMITTING ADVERSE EVENT REPORTS TO FDA
Use one of the methods below to submit voluntary adverse event reports to the FDA:
- Report Online at www.accessdata.fda.gov/scripts/medwatch/index.cfm?action=reporting.home
- Consumer Reporting Form FDA 3500B. Follow the instructions on the form to either fax or mail it in for submission. For help filling out the form, see MedWatchLearn. The form is available at www.fda.gov/downloads/aboutFDA/reportsmanualsforms/forms/ucm349464.pdf
- Call FDA at 1-800-FDA-1088 to report by telephone
- Reporting Form FDA 3500 commonly used by health professionals. The form is available at www.fda.gov/downloads/aboutFDA/reportmanualsforms/forms/ucm163919.pdf